We have a growing consumer awareness of food selection. The interest in organic foods is growing[i], the demand for fast food declining.[ii] So why is the awareness of the inherent challenges in nutritional supplement consumption so low? Why are you – and the majority of people – willing to do what everyone else is doing in the blind faith and trust that the supplement has what the label says it has, and does not contain any toxic or otherwise unhealthy material?
Let me summarize it this way – as long as ‘everyone’ is currently using it, the packaging looks great, and the nutritional labeling appears to be stacked full of what you were looking for, it’s good to go! Take the supplement, no further questions needed.
This may seem a bit too blasé in text, however tell me if this isn’t an accurate description of how the majority think. I suggest it is.
And what categories of nutritional supplement are the most exposed to ‘challenges’? According to a 2015 statement by the US Food and Drug Administration (FDA)
“They’re most common in weight loss supplements, products that promise to boost sexual performance, and bodybuilding products, [FDA spokesperson Lyndsay] Meyer Meyer said”.[iii]
How real is the problem? According to a 2015 study published in the New England Journal of Medicine, an estimated 23,000 emergency department visits in the United States every year are attributed to adverse events related to dietary supplements. Note that the categories of supplements where the greatest risks were was similar to those quoted by the FDA spokesperson (see Table).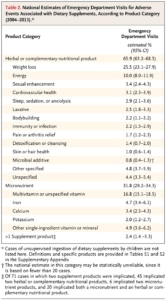 [iv]
[iv]
So where specifically do the risks lie? Here are
- Manufacturing standards
- Misleading products and product claims
- The sale of illegal supplements
- Supplements containing drugs
- Raw material selection and contamination
- Truth in labeling
- Outsourcing manufacturing
- All of the above
The following provides further insights into each of these seven areas of risk.
1. Manufacturing standards
The manufacturing of nutritional supplements in the US has been described as ‘lightly regulated’.
Yet these pills undergo limited scrutiny by regulators. The FDA treats them like food — not like drugs — under the Dietary Supplement Health and Education Act of 1994. Supplement manufacturers can put a wide variety of claims on their bottles, so long as there’s at least some research to back them up and so long as they’re honest about their ingredients. There’s no requirement for manufacturers to specify the quantity of ingredients or to warn consumers about potential side effects. What’s more, there are no regulators checking to make sure these manufacturers are telling the truth about what’s in their products before they hit store shelves.
To remove a supplement from the market, the FDA first has to prove that it’s not safe — which is what happened in the case of OxyElite Pro. This is basically the opposite of how pharmaceuticals are regulated. There, drugmakers need to prove their medicines are safe and effective through high-quality scientific studies before they ever reach consumers.[v]
Put simply, unlike pharmaceutical drug manufacturing, the FDA does not have premarket approval on supplements. Instead they have post-market oversight on supplements. They have to monitor the landscape and prove issues exist. This opens the door for a ‘it’s easier to seek forgiveness than to get approval’ approach, and you, the consumer, are in the middle of this mess.
The limitations of this lower standard of manufacturing compared to drugs is highlighted by the following:
The passing of the Dietary Supplement Health and Education Act in 1994 permitted dietary supplement manufacturers to bring to market products labeled as supplements without the scrutiny required of pharmaceuticals.
This lack of oversight has permitted the introduction of numerous supplement products, often containing unapproved active pharmaceutical ingredients, into the marketplace, which has led to harm, as was exemplified by the use of the supplement Pai You Guo.7
In these situations, it is incumbent on the FDA to contact the manufacturer of the supplement to trace the source of the product, and initiate a recall. However, a recent investigation by the Office of the Inspector General determined that the FDA does not possess accurate contact information for 20% of supplement manufacturers.8 This may explain why our study found a large discrepancy between the number of adulterated supplements reported by the FDA and the number that were actually recalled. .[vi]
As a result, this is what is often seen, as reported by Dr. Daniel Fabricant, who heads the FDA’s division of Dietary Supplement Programs:
Sixteen nationwide recalls and warnings have been issued in the past month and a half, including vitamins manufactured by Mira, which contained the risky steroids dimethazine, dimethyltestosterone and methasterone. More than 3,000 products were recalled nationwide last year.
Written product recipes at numerous supplement companies are nonexistent, Fabricant said, and many recipes — known as master manufacturing records — are apparently cobbled together when owners learn that government inspectors are on their way.
Worse, drums in which products are mixed are not always appropriately cleaned, Fabricant added, and in some firms these vessels are pitted — damaged — possibly from age and/or overuse.
Debris left from previous batches sometimes winds up in newly made products, he said.
Too often, dangerous drugs of all kinds — from male sexual enhancement compounds to weight-loss medications — are turning up in vitamins and other supplements nationwide. [vii]
2. Misleading products and product claims
The supplement history has a long history of deceit.
In 1989 Nautilus founder Arthur Jones was quoted as saying:
“I am not a bullshit artist, and under no circumstances am I going to get involved in the so-called health food market, which is one vast con game.”[1]
Arthur Jones tells a great story in his 2004 autobiography titled ‘And God Laughs’[2] about a veterinarian who was pestering him about the secret to the success of the 1973 Colorado Experiment, where Casey Viator allegedly put on an incredible amount of muscle mass in only four weeks. Jones tells him ‘the secret’, which reminds me a lot of my chapter titled ‘Colates’ from my 2010 book Barbells and Bullshit. Jones went on to write:
But if I had ever published that elephant shit story as fact in a muscle magazine, the bodybuilders would buy it by the ton, at any price; and the worse it tasted the better they would like it. I also told that veterinarian that the elephant shit always made you very sick, but he assured me that he was more than willing to put up with that.
And if I had published that story as fact, within a few weeks Weider would have been offering what he would have claimed was the only source of pure elephant shit.
US Government regulatory action against false claims in the supplement industry can be traced back at least to the 1960s (and probably earlier), when Peary Rader (Ironman Magazine), Bob Hoffman (York Barbell) and then the Weiders (1970s) were all targets of the US Food and Drug Administration.[3]
It appears not much has changed in the near half a century since.
According to the 2010 article explaining labeling and product claims in the US supplement market:
“….The impact and consequences of the Dietary Supplement Health and Education Act of 1994 (DSHEA)… Briefly, the DSHEA is an amendment to the U.S. Federal Food, Drug and Cosmetic Act that establishes a regulatory framework for dietary supplements. It effectively excludes manufacturers of these products from virtually all regulations that are in place for prescription and over-the-counter drugs.”[viii]
According to the US Food and Drug Administration (FDA) themselves:
“Generally, manufacturers do not need to register their products with FDA nor get FDA approval before producing or selling dietary supplements. Manufacturers must make sure that product label information is truthful and not misleading. FDA’s post-marketing responsibilities include monitoring safety, e.g. voluntary dietary supplement adverse event reporting, and product information, such as labeling, claims, package inserts, and accompanying literature. The Federal Trade Commission regulates dietary supplement advertising.”[ix]
Basically say what you want and unless someone complains or by some other less likely situation the FDA finds out you are ‘embellishing’ at best, downright lying at worst.
Manufacturers can put virtually any claim on a supplement, without any requirement to provide persuasive clinical evidence, as long as it’s accompanied by the Quack Miranda Warning: “These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.” Disease treatment claims are not permitted, but are typically restated as permissible “structure/function” claims, implying an ability to improve the structure or function of the body only….
There are essentially no pre-marketing requirements before selling products. Once available for sale, there is little ability for the FDA to issue cease-sale orders and recalls. Regulators can block the sale of products only after significant problems have been identified (i.e., ephedra)…
The regulation of marketing claims is effectively left to the Federal Trade Commission (FTC), which can prosecute manufacturers for fraud.” [x]
In 2015 a sweeping multi-agency federal investigation in the US has resulted in a slew of criminal and civil charges being brought against more than 100 companies that either make or market supposed dietary supplements for selling products that allegedly contain ingredients other than those listed on the label, or products that make unsubstantiated health or disease-treatment claims.[xi]
The Justice Department unsealed a criminal indictment against USPlabs of Dallas and a number of the company’s executives and employees. It charges, among other things, that they conspired to import a synthetic stimulant made in a Chinese chemical factory and then used it in products they advertised as containing all-natural plant extracts.
The indictment also alleges that USPlabs knew some of their products could cause liver damage, yet sold them anyway — in particular, a product called OxyElite Pro.[xii]
At the center of the sweep is USPlabs, a company based in Dallas that sold the best-selling workout supplements Jack3d and OxyElite Pro, which contains the amphetamine-like stimulant dimethylamylamine, or DMAA. On Tuesday, federal prosecutors brought criminal charges against USPlabs and six of its executives related to the sale of those products.[xiii]
The United States filed a civil complaint against Riddhi USA Inc. of Ronkonkoma, New York, and its owner and President Mohd M. Alam to prevent the distribution of adulterated and misbranded dietary supplements in violation of federal law, the Department of Justice announced today.[xiv]
3. The sale of illegal supplements
As there is no pre-marketing requirements before selling the products, there is a pattern of companies taking even illegal substances to the market, in the hope that they would be caught.
The following is an example of a large distributor being exposed for allegedly selling illegal supplements. Most of us have shopped here:
GNC Holdings Inc, the largest global dietary supplement retailer, has agreed to pay $2.25 million to avoid federal prosecution over its alleged sale of illegal dietary supplements, the U.S. Department of Justice said on Wednesday.[xv]
The following is an example of a smaller distributor being exposed for allegedly selling illegal supplements. The risks of mail order supplements!
Twelve years after FDA prohibited the sale of dietary supplements containing ephedrine alkaloids (ephedra), a New York man has been found guilty of violating the ban.
Following a three-day trial, a jury in Atlanta convicted Chenhsin Chan (aka Paul Chan) on 30 felony counts in connection with the online sale of dietary supplements containing ephedrine, the U.S. Department of Justice (DOJ) announced last week.
Chan of Elmhurst, New York was convicted on 10 counts of mail fraud, 10 counts of introducing adulterated food into interstate commerce, five counts of knowingly distributing a listed chemical without obtaining the required registration, and five counts of money laundering.
The 44-year-old Chan sold more than US$4.5 million in dietary supplements containing ephedrine alkaloids, but the jury forfeited assets he purchased with funds from the crimes, including a New York property that had been purchased for $950,000, a Mercedes Benz, a Lamborghini Gallardo and more than $666,000 in proceeds from the illegal activities, the DOJ noted in a press release.[xvi]
4. Supplements containing drugs
In the ideal world when you buy and ingest a supplement you would hope there are no surprises in the contents, especially unknown drugs.
From January 1, 2004, through December 19, 2012, 465 drugs were subject to a class I recall in the United States. Just over one-half (237 [51%]) were classified as dietary supplements as opposed to pharmaceutical products (Table). Most recalls occurred after 2008 (210 [89%]). Supplements marketed as sexual enhancement products (95 [40%]) were the most commonly recalled dietary supplement product, followed by bodybuilding (73 [31%]) and weight loss products (64 [27%]). Unapproved drug ingredients (237) accounted for all recalls. Fifty-seven recalled products (24%) were manufactured outside of the United States. There were 147 recalls (62%) that involved units distributed internationally. No adverse events related to recalled drugs were noted in the Enforcement Reports.
The FDA Tainted Supplement Report listed 332 adulterated products since December 2007. Only 222 of these products (69%) were recalled by the FDA.[xvii]
Placing drugs in supplements, even if only for a short time, is a method that has been spoken about in the industry for decades. The rumor was this was a technique used by early supplement manufacturers post the arrival of steroids in the 1960’s to get early market traction with their product users who were of the impression they were simply buying a supplement product.
The following supports this story. There is some suggestion that manufacturers intentionally add the drugs:
A report in the Journal of the American Medical Association in April noted that potent drugs are sometimes purposely added to supplements to increase strength, usually weight loss remedies and sleep aids. [xviii]
Now if you are a drug tested athlete, or at least a drug tested athlete in a sport that does real and transparent testing, the risk of drugs in your supplements is an additional concern.
These risks are summarized in a 2017 paper as below:
An example of the presence of doping substances in supplements can be seen in the study published in 2003 by Geyer et al., where 94 of the 634 supplements analyzed (14.8%) had prohormones that were not mentioned on the label. More current is the study by Judkins et al. in which, of the 58 supplements analyzed, 25% contained low levels of contaminating steroids and 11% were contaminated with stimulants. These data have led to the investigation of contamination in different food supplements; in most of them, small quantities of banned substances have been found, due to cross-contamination during manufacturing, processing, or packaging. In some cases, this contamination was not intentional and was due to poor quality control, but in others the adulteration of the substance was intentional [10]. In the United States (US), the Food and Drug Administration (FDA), broadly speaking, regulates quality, and the Federal Trade Commission supervises the marketing and advertising of dietary supplements. However, according to the Dietary Supplement Health and Education Act (DSHEA), dietary supplements, including nutritional ergogenic aids, that are not intended to diagnose, treat, cure, or prevent any disease, currently do not need to be evaluated by the FDA prior to their commercialization.[xix]
In my experience very few athletes succeed in a defense of blaming their nutritional supplement for the positive whereby their sentence is reduced. Examples of these minority include:
- In 2008 US swimmer Jessica Hardy failed a drug test (allegedly for the banned drug clenbuterol) and was banned for four years. When the arbitrators found that Jessica Hardy’s positive test was caused by a contaminated Advocare Arginine Extreme supplement her ban was reduced to one (1) year. Hardy subsequently took civil action against Advocare, and Advocare reciprocated in like way.[xx]
- In 2003 US swimmer Kicker Vencill failed a drug test (allegedly for the banned drug 19-norandrosterone) and was banned for four (4) years. After appeal the arbitrators accepted the positive result was caused by an inadvertent ingestion and reduced his ban to two (2) years. Following that Vencill won a civil claim when a jury ruled unanimously that a multivitamin taken by Kicker Vencill was contaminated with steroid precursors and was responsible for his positive test. Jurors awarded damages of $578,635 against the manufacturer against Ultimate Nutrition of Farmington, Connecticut.[xxi]
- Lyman Good, UFC fighter, was tested positive for steroids in October 2016, and blamed his use of a product called ‘Anavite’ made by Gaspari Nutrition and sold through Vitamin Shoppe. In Oct 2017 he sued the following: Vitamin Shoppe, Gaspari Nutrition Inc., Hi-Tech Pharmaceuticals, Richard Gaspari and Gaspari and Hi-Tech CEO Jared Wheat are also named as defendants. According to his affidavit, his suspension was later reduced when a lab detected the steroid in an unopened bottle of Anavite.[xxii] [xxiii]
The following summary of drugs found in nutritional supplements was published in 2016 that lists 850 US supplements that allegedly contain drugs.
Now you can see if your favorite supplement has been flagged by health authorities. We used data from the FDA and the Department of Defense, as well as published studies from scientific journals and court documents, to create a searchable database of dangerous supplements. All of the products listed below have been found to contain hidden drugs.[xxiv]
Here are some of the categories of drugs found in these supplements in the above collation: [xxv]
Appetite suppressants – Hidden drugs found in supplements in our database: sibutramine and its analogs (deisobutyl-benzylsibutramine, desmethyl sibutramine, didesmthyl sibutramine, n-desmthylsibutramine, n-di-desmethylsibutramine), cetilistat, fenfluramine, lorcaserin, rimonabant.
The government removed sibutramine from the market in 2010 for safety reasons… Sibutramine was the most popular drug on our database: 240 products, mostly for weight loss, contained this drug or one of its derivatives.
Laxatives – Hidden drugs found in supplements in our database: phenolphthalein
Phenolphthalein is a laxative no longer approved for sale in the US… Sixty-six supplements in our database contained phenolphthalein, most of them marketed for weight loss.
Muscle relaxants – Hidden drugs found in supplements in our database: chlorzoxazone, methocarbamol
A number of supplements marketed as arthritis and joint pain relievers have been pulled from the market for containing illegal drugs.
Sexual enhancers – Hidden drugs found in supplements in our database: sildenafil, tadalafil, vardenafil, and their analogs (acetildenafil, aidenafil, aminotadalafil, benzamidenafil, dapoxetine, desmethyl carbondenafil, dimethyl sildenafil, dimethylacetildenafil, dimethylsildenafilthione, hydroxyhomosildenafil, hydroxylthiohomosildenafil , hydroxythiohomosildenafil, noracetildenafil, piperadino vardenafil, propoxyphenyl sildenafil, sulfoaildenafil/thioaildenafil, sulfoaildenafil methanesulfonate, sulfohomosildenafil, sulfohydroxyhomosildenafil, sulfosildenafil, thiomethisosildenafil); dapoxetine
Sildenafil is the active ingredient in the prescription drug Viagra. It’s been found in hundreds of supplements (including 159 on our database). We also found dapoxetine in eight supplements in our database.
Anti-anxiety drugs – Hidden drugs found in supplements in our database: picamilon
Used in Russia to treat various neurological conditions, the synthetic drug has never been approved for sale in the US but has been found in many brain-enhancing supplements here unbeknownst to consumers.
Antidepressants – Hidden drugs found in supplements in our database: fluoxetine, doxepin
Seven of the weight loss supplements in our database contained fluoxetine, which is the active drug in the prescription antidepressant Prozac. Fluoxetine is a selective serotonin reuptake inhibitor (SSRI), a type of drug used to treat depression, obsessive-compulsive disorder, and panic disorder. You’d have no idea you were taking an SSRI in your weight loss supplement. “SSRIs have been associated with serious side effects including suicidal thinking, abnormal bleeding, and seizures,” an FDA warning letter reads. “In patients on other medications for common conditions (aspirin, ibuprofen, or other drugs for depression, anxiety, bipolar illness, blood clots, chemotherapy, heart conditions, and psychosis), ventricular arrhythmia or sudden death can occur.”
Diuretics – Hidden drugs found in supplements in our database: bumetanide, furosemide
Some weight loss supplements contain prescription-strength diuretics.
Stimulants – Hidden drugs found in supplements in our database: BMPEA, DMAA, DMBA, DEPEA, ephedrine, ephedrine alkaloids, fenproporex
BMPEA, DMAA, and DMBA were hidden ingredients in many popular muscle-building and fat-burning supplements….DMAA was banned in the US, UK, and several other countries because it has been linked to strokes, heart failure, and sudden death. Yet it was the second most popular drug on our database, appearing in 110 products on our database. DMBA is a synthetic version of DMAA …Similarly, BMPEA was never approved as a pharmaceutical, so it has never been studied in humans — and, again, it’s another common supplement ingredient.
Anabolic steroids – Anabolic steroids lurked in 81 products on our database. Most of them were marketed to men, promising to help build muscle fast.
Anti-inflammatory drugs – Hidden drugs found in supplements in our database: diclofenac, ibuprofen, indomethacin, naproxen, phenylbutazone.
Other – Hidden drugs found in supplements in our database: anthistamines (chlorpheniramine, cyproheptadine), phenytoin (anticonvulsant), chlorpromazine (antipsychotic), aromatase inhibitor, propranolol (beta blocker), nefopam (non-opioid pain relief)
5. Raw material selection and contamination
The next step to consider is the selection of the raw material. In essence you are relying on the integrity and values of the company to select safe and effective raw materials, free from contamination.
How is this working out?
In 2010 US based Consumer Reports tested 15 popular protein supplements and concluded:
All of the drinks in our tests had at least one sample containing one or more of these contaminants: arsenic, cadmium, lead and mercury. For most drinks we tested, levels were in the low to moderate range, when we could detect them. But with three of the products we tested, consumers who have three servings daily could be exposed to levels of one or two of these contaminants that exceed the maximum limits proposed by U.S. Pharmacopeia, the federally recognized authority that sets voluntary standards to cover dietary supplements.[xxvi]
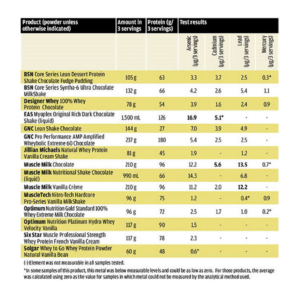
The worst offending product? EAS Myoplex Original Rich Dark Chocolate Shake.
 In 2018 the Denver-based Clean Label Project used the independent analytical chemistry laboratory Ellipse Analytics to test 134 of the top selling (based on Nielsen and Amazon.com best-selling lists) animal- and plant-based protein powders.[xxvii] Clean Label selected and purchased the powders from retail store shelves and from online sources. The products were screened for over 130 toxins including heavy metals, BPA, pesticides, and other contaminants with links to cancer and other health conditions.
In 2018 the Denver-based Clean Label Project used the independent analytical chemistry laboratory Ellipse Analytics to test 134 of the top selling (based on Nielsen and Amazon.com best-selling lists) animal- and plant-based protein powders.[xxvii] Clean Label selected and purchased the powders from retail store shelves and from online sources. The products were screened for over 130 toxins including heavy metals, BPA, pesticides, and other contaminants with links to cancer and other health conditions.
-
Of the 134 products tested, 53 were found to have “substantially elevated” levels of the following heavy metals – Lead, Mercury, Cadmium, Arsenic and BPA.
-
Of the whey-based protein powders, about 10 percent of contained lead levels above health guidelines.
-
Of the plant-based protein powders.75 percent had measurable levels of lead.
-
Each contained on average twice the amount of lead per serving as other products.
-
In addition to lead, the plant powders in several cases contained mercury, cadmium and arsenic above health-based guidelines.
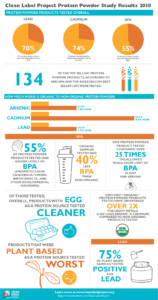
The five products that received the poorest overall scores in this test were:

In summary:
Unfortunately, federal regulations do not generally require that protein drinks and other dietary supplements be tested before they are sold to ensure that they are safe, effective and free of contaminants. [xxviii]
As a result ‘most consumers don’t realize their lives are on the line before the government steps in’:
“No supplements are prescreened for efficacy and safety by the government,” said Bryn Austin, a professor in the department of Social and Behavioral Sciences at Harvard T.H. Chan School of Public Health. “The FDA is reactive. Because of the way Congress ties their hands, they have to wait until there’s serious harm — deaths, injury, liver damage, transplants. Most consumers don’t realize their lives are on the line before the government steps in.” [xxix]
6. Truth in labeling
In 2013 ConsumerLab.com found problems with the quality of five the 16 protein products it selected for testing and confirmed these findings in a second independent laboratory:[xxx]
- A protein powder from a popular brand was missing 16 grams of protein per scoop — the majority of the protein it promised. Instead, it contained an extra 16 grams of carbohydrates (including an extra 3 grams of sugar)
- A powdered meal replacement shake was contaminated with 12.7 mcg of lead per serving (far more than permitted in California without a warning label)
- A popular protein energy meal with spirulina had an extra 6.7 grams of carbohydrates (including an extra 4 grams of sugar) and an additional 25.7 calories per serving
- A protein powder — from a “GMP certified” facility — claiming “0” cholesterol really had 10.2 mg
- A protein supplement claiming 5 mg of cholesterol actually had 14.2 mg
In 2015 the New York State attorney general’s office accused four national retailers on Monday of selling dietary supplements that were fraudulent and in many cases contaminated with unlisted ingredients.[xxxi]
The authorities said they had run tests on popular store brands of herbal supplements at the retailers — Walmart, Walgreens, Target and GNC — which showed that roughly four out of five of the products contained none of the herbs listed on their labels. In many cases, the authorities said, the supplements contained little more than cheap fillers like rice and house plants, or substances that could be hazardous to people with food allergies.
At GNC, for example, the agency found that five out of six samples from the company’s signature “Herbal Plus” brand of supplements “were either unrecognizable or a substance other than what they claimed to be.” In pills labeled ginkgo biloba, the agency found only rice, asparagus and spruce, an ornamental plant commonly used for Christmas decorations.
At Target, the agency tested six herbal products from its popular “Up and Up” store brand of supplements. Three out of six – including ginkgo biloba, St. John’s wort and valerian root, a sleep aid – tested negative for the herbs listed on their labels. But the agency did find that the pills contained powdered rice, beans, peas and wild carrots.
Here are the products that were analyzed by the attorney general, along with the test results that were described in cease-and-desist letters that the agency sent to the four retailers.
In 2016 further tests were conduced by ConsumberLab.com including 27 protein supplements — 14 selected by ConsumerLab.com and 13 others that passed voluntary certification testing – of which 28% failed quality tests. The reports findings included:[xxxii]
- Two protein powders contained more cholesterol than claimed. In fact, one which did not list any cholesterol actually had 16.5 mg per serving
- One protein powder contained 181.4 mg more sodium than listed
- Another product contained 70 mg more sodium than listed and was contaminated with cadmium, a toxic heavy metal
7. Outsourcing manufacturing
In 2003 the AustralianTherapeutic Goods Administration (TGA) suspended the licence held by Pan Pharmaceuticals Limited of Sydney to manufacture medicines, for a period of six months, because of serious concerns about the quality and safety of products manufactured by the company.[xxxiii]
The suspension follows audits of the company’s manufacturing premises, which revealed widespread and serious deficiencies and failures in the company’s manufacturing and quality control procedures, including the systematic and deliberate manipulation of quality control test data. The licence has been suspended in order to urgently address the safety and quality concerns posed by the multiple manufacturing breaches. Where the quality of a medicine cannot be certain, neither can the safety or effectiveness of that medicine.
Due to the serious and widespread nature of the manufacturing problems identified and following expert advice regarding potential risks, the TGA has taken the decision to recall all batches of medicines manufactured by Pan Pharmaceuticals Ltd since 1 May 2002 and that are being supplied on the Australian market.
219 products manufactured and supplied in Australia by Pan Pharmaceuticals Limited have been identified for immediate recall. These products have been cancelled from the Australian Register of Therapeutic Goods for quality and safety reasons. The company has also had its approval to supply its range of export products (approximately 1650) cancelled.
In addition, a further, larger recall of products manufactured by Pan Pharmaceuticals Limited under contract for other sponsors is underway. The TGA has been working with the sponsors of these products to identify those for recall. Lists of these products have been published in the newspapers and details are also available on this website.
This action essentially emptied all chemists (drug-stores) in Australia of nutritional supplements, creating a national shortage. It also made it very clear to astute consumers that they may have thought they were buying different brands, in reality most were being made in the same manufacturing facility.
While we all have our favorite brands of dietary supplements, the brands may not be as distinct as we think from a manufacturing perspective. Dietary supplement manufacturing spans a wide spectrum. One of the most common manufacturing methods is for a finished product company to contract the manufacturing to another entity. This practice is referred to as contract manufacturing or outsourced manufacturing. Contract manufacturers have been part of the dietary supplement industry from its inception, when most finished product companies were retailers and not manufacturers.[xxxiv]
There are issues arising when the finished product company sub-contracts the manufacturing process:
The use of contract manufacturers also presents some challenges. The finished product company bears ultimate responsibility for the quality of the products that bear their label. This means that the finished product company has to abide by cGMP regulations themselves and must also ensure that their contract manufacturers are compliant as well.[xxxv]
In an already low-regulated and problematic industry, another ‘cog’ in the process provides further potential complications of accountability and quality assurance. The cost to establish in-house manufacturing facilities is a luxury only a small percentage of supplement companies have.
8. All of the above
Many cases that have been subject to reporting by government regulatory agencies include more than of the above seven category breaches. Here’s a great and recent case example of this:
“…an eighteen count indictment against Jared Wheat, the CEO of Hi-Tech Pharmaceuticals, Inc. (Hi-Tech), a supplement company based in Norcross, Georgia, has been unsealed. According to the superseding indictment, the current charges against Wheat include wire fraud, money laundering, introducing misbranded drugs into interstate commerce and manufacturing and distributing controlled substances, specifically Schedule III controlled anabolic steroids. There are also charges in the indictment against John Brandon Schopp, the Director of Contract Manufacturing for Hi-Tech.
The indictment alleges that Wheat, Schopp, and Hi-Tech manufactured and distributed to prospective and current customers false U.S. Food and Drug Administration (FDA) Certificates of Free Sale, good manufacturing practice (GMP) certificates, and GMP audit reports. The indictment contends that the GMP certificates and audits reports, which are supposed to come from an independent third party, were issued by PharmaTech, a company controlled by Wheat. The manufacturing and distributing controlled substances charges stem from the government’s allegations that Hi-Tech produced at least five supplements containing anabolic steroids. Anabolic steroids are a Schedule III controlled substance and require a prescription. The misbranded drug charge alleges that Wheat and Hi-Tech manufactured a supplement named Choledrene which contained lovastatin. Lovastatin is an ingredient used in statin drugs and is regulated by the FDA. Hi-Tech should never have used lovastatin as an ingredient in any supplement, and they did not include it on the list of ingredients on the bottle.[xxxvi]
The following is an interesting insight into the effectiveness of past attempts to modify behavior in this industry:
If he is found guilty, this will not be Wheat’s first time in jail. In 2014, he served two months in federal prison for failing to carry out a recall brought about the Federal Trade Commission’s claim of false advertising for Hi-Tech’s weight loss products. Wheat was released when the recall had been completed. In 2009, the FDA announced he received a two-year prison sentence after being found guilty of selling counterfeit medications online, which he claimed were made in Canada, but were actually manufactured in unsanitary conditions in Belize. An investigation by AJC listed several other legal battles between federal authorities and Wheat/Hi-Tech. [xxxvii] [xxxviii]
An irrestible opportunity to make serious money – Seeking forgiveness is easier than seeking permission
“Pssst! Wanna start a supplement company? There is sooo much money to be made! We can make up anything we want, say anything we want, and if theproverbial hits the wall we can go straight. After all, humans are so gullible. As long as we hire some great copy writers to make some very convincing long copy. We can also make an internet (and or hard copy) magazine to provide ‘third-party’ indirect endorsements!”
The global dietary supplements market is expected to reach USD 278.02 billion by 2024, according to a new report by Grand View Research, Inc. Favorable outlook towards medical nutrition market in light of increasing application for the treatment of malnutrition and cardiovascular disorders is likely to promote the market for dietary supplements.
Rising sales of sports nutrition products in the U.S. and China on account of increasing prevalence of fitness and sports at a domestic level along with new product launches is likely to have a significant impact on the industry over the projected period. The market is expected to generate revenues worth USD 37.16 billion by 2024.
Rising consumption of clinical nutrition products as a prevention medium for reducing malnutrition is expected to have a substantial impact. Furthermore, increasing prevalence of premature births on a global level is expected to promote the use of medicinal supplements over the forecast period. The market was worth USD 19.17 billion in 2015 and is projected to witness growth at a CAGR of 9.5% from 2016 to 2024.[xxxix]
Groundbreaking US female computer scientist and a Navy officer Grace Hopper[xl] (back when it was virtually impossible for a woman to succeed in either role) is commonly credited with coining the phrase that’s the mantra of a lot of 21st Century nutritional supplement entrepreneurs:
“It is easier to get forgiveness than permission.”
The following is an example of this commercial value set:[xli]
AngelList “corporate policy” is that team members should ask forgiveness, not permission. We would rather have someone do something wrong than ask permission to do it.
Now in fairness they do add:
Or better, we would rather have someone do something right and not need permission to do it. This is the most common outcome.
But when you and I can make SO much money, let’s forget about that….
Conclusion
So how widespread in the supplement industry are these ‘challenges’? According to 2013 statement by Dr. Daniel Fabricant, head the FDA’s division of Dietary Supplement Programs at the time:
“…About 70 percent of the nation’s supplement companies have run afoul of the U.S. Food and Drug Administration’s manufacturing regulations over the past five years, according to a top agency official….
Consumers are put at risk by poorly measured ingredients, uncleaned manufacturing equipment, pesticides in herbal products, supplements contaminated with illegal prescription medications — even bacteria in pediatric vitamins, recall notices and agency inspection records have shown….
While most vitamins and supplements are not harmful — and at least one vitamin brand was credited with an 8 percent reduction in cancer among men over 50 — the industry is beset by repeated recalls, manufacturing problems and adverse reactions caused by tainted products, health experts and regulatory officials say….” ”[xlii]
If you have read this entire article, and have reached this point, I would be keen to know what you are thinking. Has this changed your view point on who you can trust? Personally, the more I became aware of this challenge during the last few decades, the more I have retreated to working with companies led by people whose value for excellence, safety, and regulatory compliance I trust. After all, it is more than just the health of my family and myself – I am responsible for the lives and careers of many athletes and coaches, whose livelihoods, reputations and legacies can be extinguished with a few words – ‘positive drug test’.
Quite simply I am amazed at how much energy goes into the discussion of supplements focused almost exclusively on the manufacturers marketing and labeling claims, completely oblivious to the real challenges in nutritional supplements – the challenges raised in this article. Your beliefs are virtually useless in the absence of your awareness of the companies values, integrity, raw material selection, manufacturing process and so on.
“From California to Maine, consumers ingest pills, powders, and liquids every day, not knowing whether they are wasting money or whether they may end up harming, rather than helping, themselves,” said Benjamin Mizer, principal deputy assistant attorney general. “Unfortunately, many of these products are not what they purport to be or cannot do what the distributors claim they can do.”[xliii]
Here’s my challenge to you. Do you want to do what’s best for your short, medium and long term health? Or is your desire to conform to the power of marketing and social trends so great that you are willing to forgo your health? Because I suggest right now the latter is your dominant value. Perhaps you didn’t know any better? Now you do. It will be interesting to see what direction you take now…..
References
[1] Cited in Roach, R., 2011, Muscle, Smoke and Mirrors, Vol. 2, Author House, p. 619.
[2] Jones, A., 2004, And God Laughs, The Autobiographical Memoirs of Arthur Jones, PDA Press.
[3] Roach, R., 2011, Muscle, Smoke and Mirrors, Vol. 1, Author House, p. 394.
[i] https://www.organicconsumers.org/news/demand-organic-food-growing-faster-domestic-supply
[ii] http://fortune.com/2015/04/22/mcdonalds-restaurants-closing/
[iii] Marcus, M., 2015, How safe are your dietary supplements?, CBS News, Nov 18 2015, https://www.cbsnews.com/news/dietary-supplements-how-safe-are-they/
[iv] Geller, A.I., et al, 2015, Emergency Department Visits for Adverse Events Related to Dietary Supplements, New England Journal of Medicine 2015, 373:1531-1540. https://www.nejm.org/doi/full/10.1056/NEJMsa1504267
[v] Belluz, J., 2015, The government is bringing criminal charges against companies that sell bogus dietary supplements, Vox, No 18 2015, https://www.vox.com/2015/11/17/9751592/dietary-supplements-justice-criminal-charges
[vi] Harel, Z., Harel, S., and Ward, R., 2013, The Frequency and Characteristics of Dietary Supplement Recalls in the United States, May 27 2013, JAMA Intern Med. 2013;173(10):929-930.https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/1678813
[vii] Ricks, D., 2013, FDA official: 70% of supplement companies violate agency rules, Newsday, Aug 16 2013, https://www.newsday.com/news/health/fda-official-70-of-supplement-companies-violate-agency-rules-1.5920525
[viii] Gavuro, S., 2010, Supplement Regulation: Be Careful What You Wish For, Science-based Medicine, Augus 5 2010, https://sciencebasedmedicine.org/supplement-regulation-be-careful-what-you-wish-for/
[ix] https://www.fda.gov/food/dietarysupplements/default.htm
[x] Gavuro, S., 2010, Supplement Regulation: Be Careful What You Wish For, Science-based Medicine, Augus 5 2010, https://sciencebasedmedicine.org/supplement-regulation-be-careful-what-you-wish-for/
[xi] Moran, C., 2015, Feds File Criminal, Civil Cases Against More Than 100 Supplement Companies, Consumerist, 17 Nov 2015, https://consumerist.com/2015/11/17/feds-file-criminal-civil-cases-against-more-than-100-supplement-companies/
[xii] Swetlitz, I., 2015, Dietary supplement manufacturers face flurry of federal charges, Statnews, Nov 17 2015, https://www.statnews.com/2015/11/17/supplements-fda-criminal-charges/
[xiii] Latman, P., and O’Connor, A., 2015, Makers of Nutritional Supplements Charged in Federal Sweep, Well, Nov 17 2015
https://well.blogs.nytimes.com/2015/11/17/federal-officials-target-dietary-supplement-makers/
[xiv] Department of Justice (DOJ), 2017, United States Files Enforcement Action Against Long Island Company and Its Owner to Prevent Distribution of Adulterated and Misbranded Dietary Supplements, Press Release, 23 Oct 2017, https://www.justice.gov/opa/pr/united-states-files-enforcement-action-against-long-island-company-and-its-owner-prevent
[xv] Lynch, S., 2016, GNC settles dietary supplements case with U.S. government, Reuters, 16 Dec 2016, https://www.reuters.com/article/us-gnc-hldg-settlement-idUSKBN13W2B2
[xvi] Natural Products Insider, 2016, Dietary Supplement Marketer Convicted of Selling Ephedra Years After FDA Ban, June 03, 2016, https://www.naturalproductsinsider.com/litigation/dietary-supplement-marketer-convicted-selling-ephedra-years-after-fda-ban
[xvii] Harel, Z., Harel, S., and Ward, R., 2013, The Frequency and Characteristics of Dietary Supplement Recalls in the United States, May 27 2013, JAMA Intern Med. 2013;173(10):929-930.https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/1678813
[xviii] Ricks, D., 2013, FDA official: 70% of supplement companies violate agency rules, Newsday, Aug 16 2013, https://www.newsday.com/news/health/fda-official-70-of-supplement-companies-violate-agency-rules-1.5920525
[xix] Martinez-Sans, J.M., et al, 2017, Intended or Unintended Doping? A Review of the Presence of Doping Substances in Dietary Supplements Used in Sports, A revew, Nutrients, 4 Oct 2017
[xx] Swimming Worldm 2009, Jessica Hardy Suspension Reduced to One Year, Supplement Ruled as Contaminated; USA Swimming Releases Statement; USADA Press Release; AdvoCare Disputes Findings – Updated, 4 May 20109, https://www.swimmingworldmagazine.com/news/jessica-hardy-suspension-reduced-to-one-year-supplement-ruled-as-contaminated-usa-swimming-releases-statement-usada-press-release-advocare-disputes-findings-updated/
[xxi] ESPN, 2005, Vencill was suspended two years, missed Olympics, 14 May 2005, http://www.espn.com.au/skiing/news/story?id=2059714
[xxii] Rummell, N., 2017, Pro Fighter Blames Supplements for Failed Drug Test, Courthouse News Service Oct 19 2017, https://www.courthousenews.com/pro-fighter-blames-failed-drug-test-supplements/
[xxiii] Earlier in 2017 the U.S. Anti-Doping Agency conducted its own analysis of Anavite and found it contained 1-andro. The agency now warns athletes not to use it, noting that Gaspari Nutrition has received numerous warnings from the Food and Drug Administration since 2014 regarding claims that its products are adulterated with potentially dangerous ingredients.
[xxiv] Belluz, J., and Oh, S., 2016, Unregulated, https://www.vox.com/a/supplements
[xxv] Belluz, J., and Oh, S., 2016, Unregulated, https://www.vox.com/a/supplements
[xxvi] Consumer Reports, 2010, How about some heavy metals with that protein drink?, June 2 2010, https://www.consumerreports.org/cro/news/2010/06/how-about-some-heavy-metals-with-that-protein-drink/index.htm
[xxvii] Clean Label Project, 2018, 2018 Protein Powder Study, https://www.cleanlabelproject.org/protein-powder/
[xxviii] Consumer Reports, 2010, How about some heavy metals with that protein drink?, June 2 2010, https://www.consumerreports.org/cro/news/2010/06/how-about-some-heavy-metals-with-that-protein-drink/index.htm
[xxix] Marcus, M., 2015, How safe are your dietary supplements?, CBS News, Nov 18 2015, https://www.cbsnews.com/news/dietary-supplements-how-safe-are-they/
[xxx] ConsumerLab.com, 2013, 31% of Protein Powders and Drinks Fail Tests by ConsumerLab.com, June 2 2013, https://www.consumerlab.com/news/Protein_Powders_Reviewed/06_11_2013/
[xxxi] O’Connor, A., 2015, What’s in Those Supplements?, Well, https://well.blogs.nytimes.com/2015/02/03/sidebar-whats-in-those-supplements/
[xxxii] ConsuberLab.com, 2016, Protein powders, shakes and drinks review, June 10 2016, https://www.consumerlab.com/reviews/Protein_Powders_Shakes_and_Drinks_Including_Nutrition_Diet_Meal-Replacement_and_Sports_Endurance_Recovery/NutritionDrinks/
[xxxiii] Australian Government, Department of Health, Therapeutic Goods Adminstration, Pan Pharmaceuticals Limited: Regulatory action & product recall information, April 28 2003, https://www.tga.gov.au/product-recall/pan-pharmaceuticals-limited-regulatory-action-product-recall-information
[xxxiv] Alschuler, L., 2011, Who Makes These Dietary Supplements, Anyway?, Natural Medicine Journal, Dec 2011, Vol 3(12), https://www.naturalmedicinejournal.com/journal/2011-12/who-makes-these-dietary-supplements-anyway-0
[xxxv] Alschuler, L., 2011, Who Makes These Dietary Supplements, Anyway?, Natural Medicine Journal, Dec 2011, Vol 3(12), https://www.naturalmedicinejournal.com/journal/2011-12/who-makes-these-dietary-supplements-anyway-0
[xxxvi] The Partnership for Safe Medicines, 2017, Company And Ceo Charged With Illegally Adding Scheduled Drugs To Supplements, Oct 27 2017, https://www.safemedicines.org/2017/10/company-and-ceo-charged-with-illegally-adding-scheduled-drugs-to-supplements.html
[xxxvii] The Partnership for Safe Medicines, 2017, Company And Ceo Charged With Illegally Adding Scheduled Drugs To Supplements, Oct 27 2017, https://www.safemedicines.org/2017/10/company-and-ceo-charged-with-illegally-adding-scheduled-drugs-to-supplements.html
[xxxviii] Brunkner, M., 2009, Diet Supplement king gets 50 months in prison, NBC News, Crime and Courts, February 3 2009, http://www.nbcnews.com/id/28983195/ns/us_news-crime_and_courts/t/diet-supplement-king-gets-months-prison/#.WwkvG2Xuz7Y
[xxxix] Grand View Research, 2016, Dietary Supplements Market Size Is Projected To Reach $278.02 Billion By 2024, Demand In Food & Beverage Sector : Grand View Research, Inc., San Francisco, July 18, 2016 (GLOBE NEWSWIRE), https://globenewswire.com/news-release/2016/07/18/856668/0/en/Dietary-Supplements-Market-Size-Is-Projected-To-Reach-278-02-Billion-By-2024-Demand-In-Food-Beverage-Sector-Grand-View-Research-Inc.html
[xl] https://www.facebook.com/notes/big-optimism/grace-hopper-its-better-to-ask-forgiveness-than-permission/521620811362966
[xli] Venture Hacks, 2013, Good advice for start-ups, Ask forgiveness, not permission, Feb 11 2013, http://venturehacks.com/articles/ask-forgiveness-not-permission
[xlii] Ricks, D., 2013, FDA official: 70% of supplement companies violate agency rules, Newsday, Aug 16 2013, https://www.newsday.com/news/health/fda-official-70-of-supplement-companies-violate-agency-rules-1.5920525
[xliii] Belluz, J., 2015, How 2 dietary supplement companies made $400 million off bogus weight loss products, Vox, No 19 2015, https://www.vox.com/2015/11/19/9761524/usp-labs-indictment




 In 2018 the Denver-based Clean Label Project used the independent analytical chemistry laboratory Ellipse Analytics to test 134 of the top selling (based on Nielsen and Amazon.com best-selling lists) animal- and plant-based protein powders.
In 2018 the Denver-based Clean Label Project used the independent analytical chemistry laboratory Ellipse Analytics to test 134 of the top selling (based on Nielsen and Amazon.com best-selling lists) animal- and plant-based protein powders.



